Late last year, Cannabidiol, or CBD for short was removed from the Misuse of Drugs Act as a schedule B drug. This change had little practical changes for patients because Peter Dunne had removed a lot of Class B
requirements for CBD previously (he didn't seem to have authority to push through a full and proper descheduling). The key difference between CBD and other Cannabis products is that they do not require Ministry of Health approval for each patient. For this reason their are several hundred people able to access CBD on a monthly basis now.
Despite CBD being no longer a Class B Drug, it is still scheduled as a medicine, which means it needs a prescription from a GP or specialist.
Complicating things further, no product has been approved by Pharmac, which means all CBD products are unregistered medicines. The rules around unregistered medicines are strict and are designed to stop pharmaceutical companies pushing products that are untested, or untested against a specific condition. This has resulted in an effective blackout on awareness in many quarters around what products are available as advertising is not tolerated, in fact even listing the products on a public facing website could be breaking the current rules. It is
however anticipated that next year Manufacturers will be able to advertise to Medical Professionals, but not the general public.
Other more hopeful changes that could happen are changes to make certain CBD products more freely available, ditching the requirements for a prescription. Some changes could include excluding products below a certain dose from the medicines act, or making certain products pharmacy only, or Pharmacist only, not unlike the stronger cold and flu medicines. This would bring us inline with many countries where even Japan does not restrict CBD itself.
[caption id="attachment_3409" align="alignright" width="300"]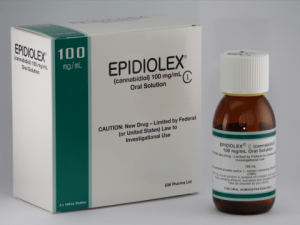 Epidiolex is the only CBD product that has gone through the entire trial process, and is now FDA approved.[/caption]
Epidiolex is the only CBD product that has gone through the entire trial process, and is now FDA approved.[/caption]
CBD has one well researched pharmaceutical product, Epidiolex, which has completed phase 3 trials for Lennox Gastaut Syndrome and Dravet Syndrome, both severe forms of epilepsy. This is the only product that would likely meet Medsafe approval to be used to treat a specific condition (on label), however other companies do have products in the trial pipeline. Epidiolex still doesn't have availabilty in New Zealand on the Horizon. For this reason any use in NZ is effectively experimental, with little in the way of dosing guidelines for specific conditions developed, further compounding difficulties for Medical Professionals.
There is good news on the horizon however, as one of the key issues to date has been affordability, due to the law changes and improved access, several companies vying to bring in products, it appears that the price of CBD is set to tumble over the next 6-12 months, going from "unobtanium" to merely unaffordable for most. These price drops will not be enough for beneficiaries, and without hard research data the likes of ACC will not be picking up the bill, but for the "working wounded" CBD will be within the realms of possibility from 2020 onward.
CBD in NZ is typically prescribed for Chronic Pain, and anxiety. It can be prescribed for Epilepsy but the effective dose needed is typically much higher, causing it to be unaffordable for most. Most Chronic Pain use is also cost limited, with daily doses typically ranging from 50-200mg per day. Of all the conditions, Anxiety is possibly the most affordable to treat, typically requiring lower doses than Chronic Pain.
If you want to know more about prescribing CBD either as a patient or a medical professional, feel free to contact us.
